Proteins flip over to reverse the spin direction of a tiny motor that drives movement.
Introduction
Salmonella enterica serovar typhimurium is a common type of bacteria best known among humans for causing food poisoning. Like many single-celled organisms, salmonella moves through its environment using special tail-like structures called flagella. This movement helps the salmonella find food and avoid danger. Each singular flagellum acts like a tiny motor, spinning to push the bacteria forward. A key feature of this motor is its ability to change the direction of its spin between clockwise (CW) and counterclockwise (CCW), allowing the bacteria to change direction and explore more efficiently.
The Strategy
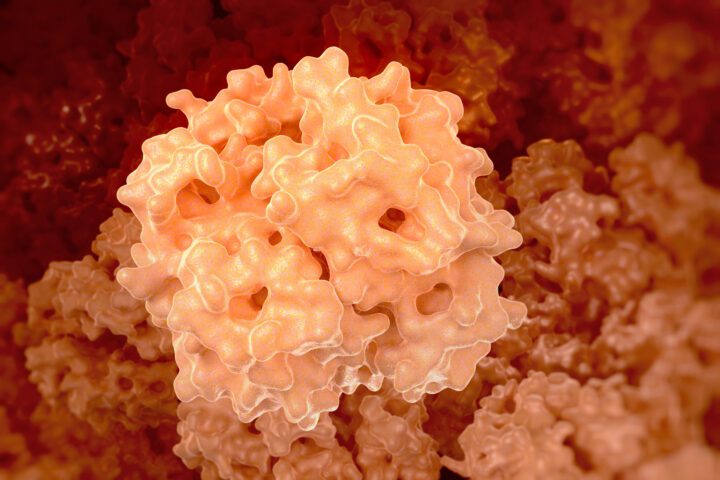
The flagellar motor of salmonella is a complex structure made up of several rings and a central rod. The C-ring (cytoplasmic ring) is especially important because it controls the direction of rotation. The C-ring contains proteins called FliG, FliM, and FliN, which work together as the motor’s “switch.” This switch can flip between two positions, allowing the flagellum to rotate either CW or CCW.
When the motor spins CCW, the bacteria swim straight. When it spins CW, the bacteria tumble and change direction. This switching mechanism is crucial for chemotaxis, as it allows the bacteria to adjust its movement in response to its environment.
The change between CW and CCW rotation involves significant structural rearrangements within the C-ring. In the CCW configuration, FliG proteins form a V-shape, creating an inner and outer ring separated by a small gap. When the motor switches to CW rotation, these proteins rotate 180 degrees, changing their interactions and the orientation of binding sites for other components called MotA and MotB. This reorientation is essential for reversing the direction of the force applied to the flagellar rod, enabling the switch of direction for the flagellum.
The force that drives the flagellum’s rotation comes from interactions between the C-ring and the MS-ring (membrane-supramembrane ring), which connects to the flagellar rod. The MS-ring translates the rotational force into movement, driving the flagellum’s spin. The structure and flexibility of these components are crucial for the motor’s function, allowing it to handle the stresses of rapid rotation and directional switching.
The Potential
Understanding how the bacterial flagellum works can inspire new ideas for advanced technology and engineering. For example, the principles of bidirectional rotation and force transmission could help design tiny, efficient motors for medical devices, like targeted drug delivery systems. Additionally, the flexibility seen in the flagellar motor might lead to the creation of materials that can change shape and function in response to different conditions.
By mimicking the natural efficiency and adaptability of the bacterial flagellum, engineers and designers can develop innovative solutions that work well with biological systems, promoting sustainability and reducing environmental impact. This approach follows the principles of , which aims to solve human challenges by learning from and copying nature’s successful strategies.
AI on AskNature
This page was produced in part with the assistance of AI, which is allowing us to greatly expand the volume of content available on AskNature. All of the content has been reviewed for accuracy and appropriateness by human editors. To provide feedback or to get involved with the project, contact us.