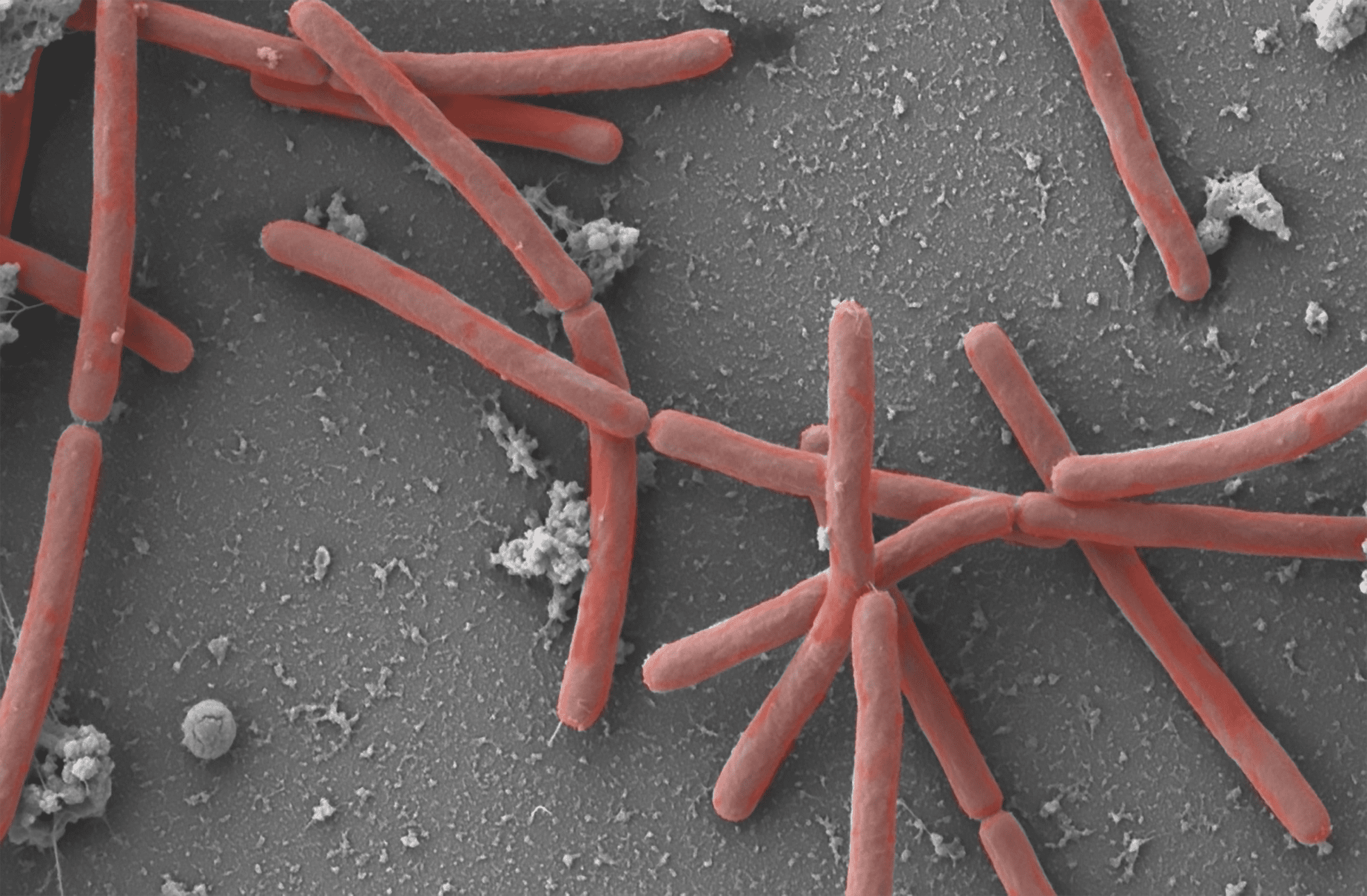
Catalyze Chemical Breakdown
Life depends upon the building up and breaking down of biological molecules. Catalysts, in the form of proteins or RNA, play an important role by dramatically increasing the rate of a chemical transformation––without being consumed in the reaction. The regulatory role that catalysts play in complex biochemical cascades is one reason so many simultaneous chemical transformations can occur inside living cells in water at ambient conditions. For example, consider the 10-enzyme catalytic breakdown and transformation of glucose to pyruvate in the glycolysis metabolic pathway.
Chemically Break Down Organic Compounds
The vast majority of biochemical assembly and breakdown processes–even by the most complex organisms–occur within cells. In fact, cells are able to perform hundreds, even thousands of chemical transformations at the same time under life-friendly conditions (ambient temperature and pressure in an aqueous environment). Part of the reason that decomposition reactions (chemical breakdown) can occur under such mild conditions is because most often, they occur in a stepwise, enzyme-mediated fashion, sipping or releasing small amounts of energy at each step. For example, the breakdown of glucose to pyruvate–and the release of energy–occurs in the 10-step enzyme catalyzed process of glycolysis.
Modify Chemical Potential
Building a dam on a flowing river creates a difference in water level on either side of the dam. The difference in water level is called a potential because it takes advantage of the abiotic tendency of water to seek its own level. Once there is an opening in the dam, water will rush from the higher level to the lower level until they become equal. The flow of water can be used to do work, like turning a turbine to generate hydroelectric power. Similarly, a chemical potential can be set up to do work. For example, in photosynthesis, the energy of a solar photon striking a leaf forces an electron to flow along the electron transport chain. As the electron passes each point in the chain, a hydrogen ion is released within the plant cell’s thylakoid membrane. As the hydrogen ions build up on one side of the thylakoid membrane, it sets up a chemical potential due to the difference in hydrogen ion concentration on either side. Just as there is an abiotic tendency for water to seek its own level on either side of the dam, there is an abiotic tendency for chemical concentration of any particular ion or molecule to “seek its own concentration level” on either side of a membrane. In photosynthesis, hydrogen ions find their way to the other side of the thylakoid membrane through a pathway created by an embedded enzyme channel. The flow of hydrogen ions through the channel power the enzyme’s chemical synthesis machinery.
Cooperate/Compete Between Different Species
From parasitism to mutualisms, there are endless interactions between organisms in nature. Interactions between species that lead to a negative outcome for one species are known as competition. Cooperation occurs when organisms work together for the benefit of each organism or species. While competition often occurs over resources or mates, the more biologists have begun to look for examples of cooperation in nature, the more they have found. For example, around 90% of plants have a beneficial partnership with fungi. Fungi provide the plant with nutrients such as nitrogen and phosphorus, in exchange for sugars from the plant.