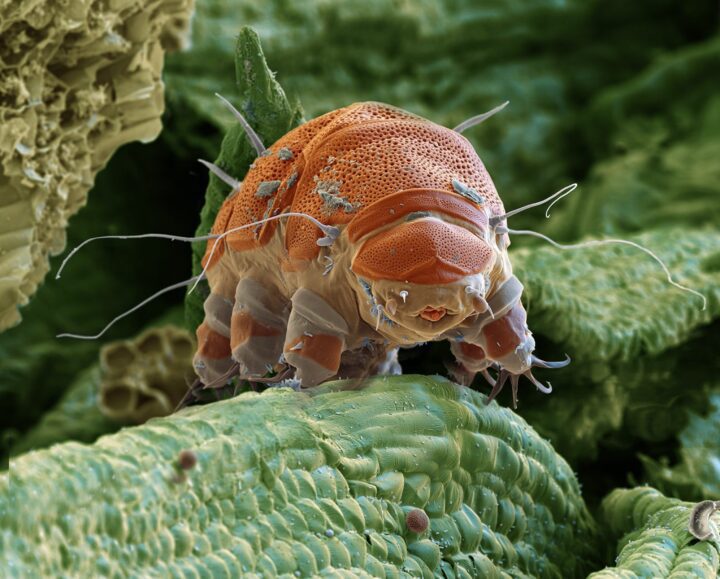
Pompeii worms tolerate the steepest temperature gradient on the planet using multiple strategies.
“Some of my colleagues have nominated the worm Alvinella pompejana (right) as the most thermally tolerant animal on Earth. Whole galleries of alvinellids live in tubes on the sides of black smokers, at the very seething heart of the vent. University of Delaware marine biologist Craig Cary has gathered data that show alvinellids living in water that’s 149o F and surviving frequent temperature spikes well above 175oF. Alvinellids–on average a half inch in diameter and about three inches long–also tolerate the steepest temperature gradient on the planet. Specimens have been found in water 140oF hotter at one end of the animal than at the other. Superheated fluids from black smokers do not mix well with ambient cold seawater, so transitions between them are abrupt. ‘Textbook biology tells us that animals can be psychrophilic [cold loving] or thermophilic [heat loving] but not both,’ says Cary. ‘I guess the alvinellids just didn’t read the textbook.’ We don’t yet know how Alvinella worms survive these extremes. The answer may lie in their behavior or in some specialized cellular biochemistry, or both.” (Lutz 2000)
“The Pompeii worm is capable of withstanding temperatures as high as 105 °C (Chevaldonne et al. 1992), and is known as being the most eurythermal metazoan (Haddad et al. 1995). Experiments carried out by Brisa et al. (2005) demonstrated that A. pompejana influences mineralization processes at the interface between the smoker wall and the ambient oceanic water. The study also indicated that A. pompejana exerts a primary control on its environment by structuring the thermal and chemical gradients, creating a mosaic of micro-environments by the construction of protective tubes. The supply of water through the tube prevents exposure to the extreme temperature spikes and high sulphide concentrations. Circumventing the large erratic changes generally associated with hydrothermal venting, A. pompejana tubes create more homogeneous chemical and thermal microniches and likely play a role in microbial diversity.
“Collagens are among the most ubiquitous s found in the animal kingdom and can be found in sponges as well as in vertebrates (Scandurra et al. 2000). Collagens are extracellular proteins with triple-helical domains (vander-Rest & Garrone 1991). Among collagens, the most homogeneous subfamily is that of fibrillar collagens (Wharton & Brown 1991). Fibrillar collagens possess a long central triplehelical domain of 330 to 340 GNN triplets, flanked by an amino-propeptide (N-pro) and a carboxyl-propeptide (C-pro). Collagens are of great importance, not only in performing cohesion between cells, but also in cell differentiation and migration (Wharton & Brown 1991; vander-Rest & Garrone 1991). Whereas the interstitial collagen of coastal polychaete worms (e.g. Arenicola marina) is denatured at 28 °C, the collagen of A. pompejana remains stable at 45 °C and is thus the most thermostable fibrillar collagen known (Gaill et al. 1995). Its collagen is adapted to the hydrothermal vent environment by its stability at higher temperatures (Gaill et al. 1995) and high pressures (Auerbach et al. 1995), and by its associated enzymatic processes, which appear to be optimized under anoxic conditions (Kaule et al. 1998).
“The molecular basis of this thermal behaviour includes the increase in proline content and in the number of stabilizing triplets, which correlate with the outstanding thermostability of the interstitial collagen of A. pompejana. Proline residues are of primary importance in performing cohesion between the chains of the triple of collagens (vander-Rest & Bruckner 1993; Prockop & Kivirikko 1995). Biochemical analysis has so far been unable to explain Alvinella‘s collagen thermostability (Gaill et al. 1995). A molecular genetic approach has been used by Sicot et al. (2000), who have cloned and sequenced a large cDNA molecule coding the fibrillar collagen of Alvinella, including one-half of the domain and the entire C-propeptide domain. For comparison, the group also cloned part of the homologous cDNA from Riftia (Pohlschroder et al. 1997). Comparison of the corresponding helical domains of these two species, together with that of the sequenced domain of the coastal lugworm Arenicola marina (Sicot et al. 1997), showed that the increase in proline content and in the number of stabilizing triplets correlates with the outstanding thermostability of the interstitial collagen of A. pompejana. Phylogenetic analysis showed that the triple helical and the C-propeptide parts of the same collagen molecule evolve at different rates, in favour of an adaptive mechanism at the molecular level.” (Islam & Schulze-Makuch 2007:207)