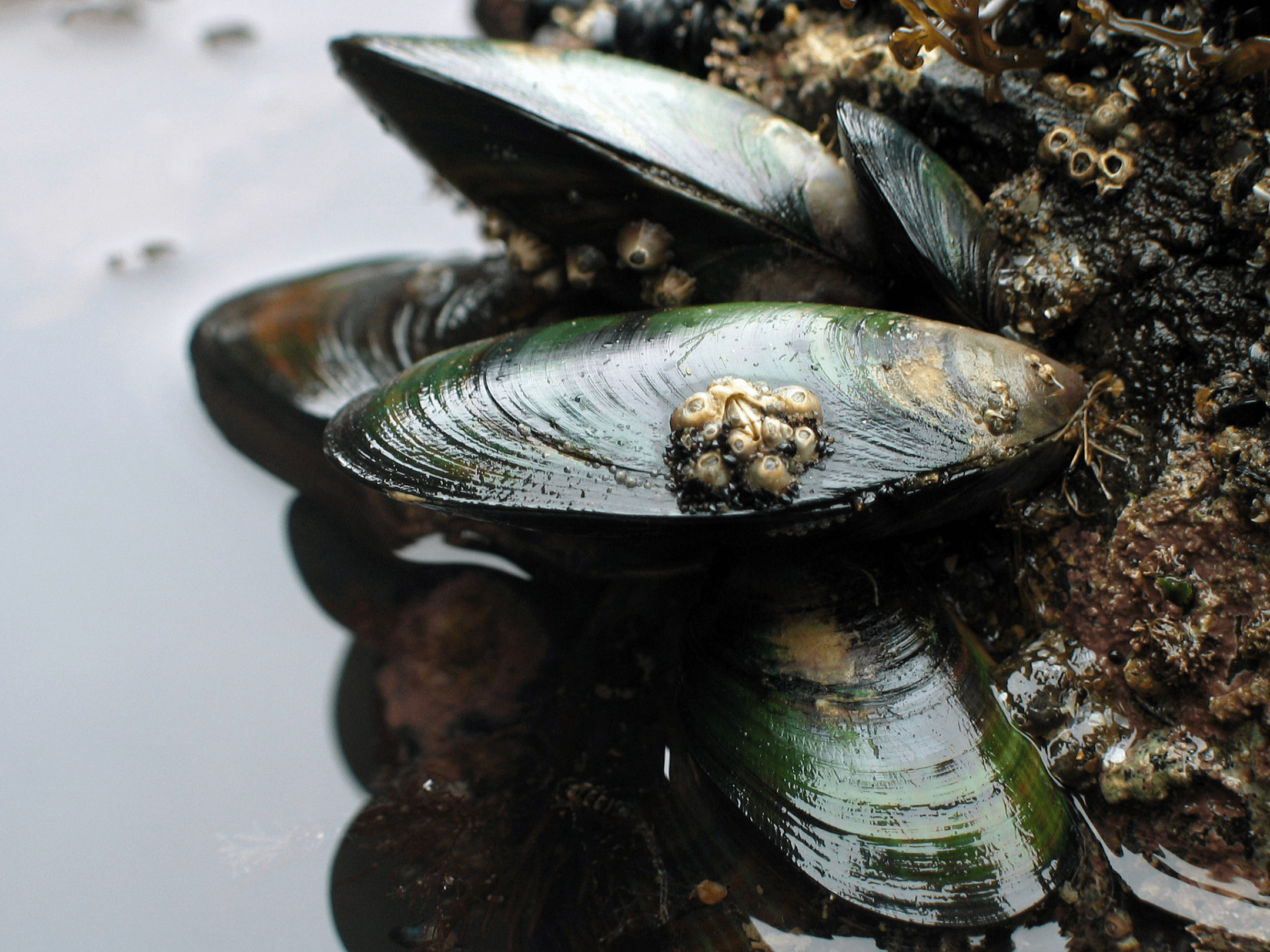
The mussel-inspired nanocellulose (MINC) coating from Pennsylvania State University uses negatively charged ions to pull rare earth elements from water while using little energy.
Benefits
- Reduced energy
- Reduced pollution
Applications
- Green power generation
- Electric vehicles
UN Sustainable Development Goals Addressed
-
Goal 6: Clean Water & Sanitation
-
Goal 7: Affordable & Clean Energy
-
Goal 9: Industry Innovation & Infrastructure
The Challenge
Similar to other rare earth elements, neodymium (Nd) is an important ingredient for powering green technologies such as wind turbines and electric vehicles that help lower our society’s environmental footprint. Paradoxically, conventional methods of extracting Nd and other rare elements from the earth consume high levels of energy that cause both air and water pollution.
Biological Model
Mussels tether themselves to underwater rocks with stringy fibers called byssal threads. Each thread has a foamy adhesive “plaque” at the end containing a mixture of s that give mussels their amazing adhesive qualities. One protein repels negatively charged ions on the surfaces of rocks, like a magnet turned the wrong way, clearing the path for another protein to latch on.
Innovation Details
The extractive coating is composed of an array of tiny nanocrystals. The hair-like tendrils of the nanocrystals are negatively charged ions, making them highly selective towards positively charged neodymium ions. The attractive force enables the coating to pull out the rare earth element from industrial wastewater similar to how a magnet would attract iron—but without using high quantities of energy.