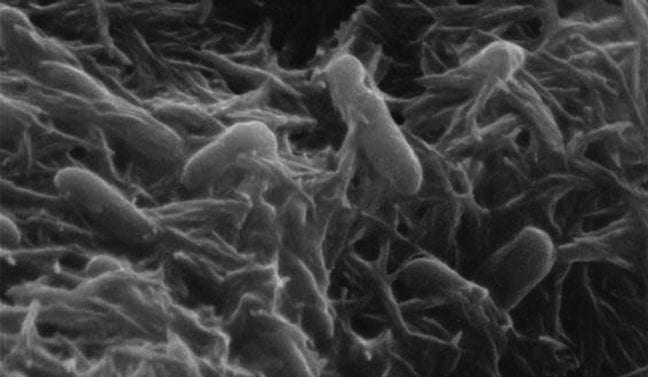
Microbes build external protein networks of "nanowires" to export electrons outside their cell walls.
Introduction
In earlier eras, single-celled organisms were seen as “simpler.” Advances in microscopy and biochemistry have shown us just how oversimplified that view is. They may be tiny, but they’re performing many of the same tasks as the largest multicellular organisms, and their size actually opens up interesting possibilities and alternatives for them.
To get energy to live and grow, some bacteria build electrical “wires” 100,000 times thinner than a human hair. They extend these nanowires outside their cell walls and create a microscopic electrical grid in the surrounding environment. The nanowires allow the bacteria to “breathe,” using metals instead of oxygen.
Living things generate energy by breaking down bigger chemical compounds into smaller ones through a series of chemical reactions known as cellular respiration. In each reaction, chemical bonds are broken, releasing electrons that are transported from compound to compound. At each step, the electrons give up a little more of their energy, which is siphoned off to do work that keeps cells alive. At the end of this cascade, the energy-reduced electrons must find a final resting place in the atomic orbit of some element or compound.
Humans and many other organisms use oxygen to accept these electrons because it is readily available in the atmosphere and can be directly absorbed or inhaled. This is known as aerobic respiration. Many bacteria, however, live deep in sediments where oxygen does not penetrate, and must use another electron acceptor. This is known as anaerobic respiration, or respiration without oxygen.
The Strategy
Metals such as iron, manganese, and arsenic—and even radioactive uranium—readily accept excess electrons, and they are commonly found in soil. But here’s the hitch: Bacteria can’t bring metals inside their cell walls, because the metals are too big, or toxic to the bacteria, or stuck to other surfaces. So the bacteria have to export their excess electrons outside, in a process known as extracellular respiration.
That’s where the nanowires come in. The bacteria use proteins to build thin structures 3 to 5 nanometers in diameter, about the size of a strand of human DNA. These nanowires extend beyond the bacterial cell walls, where they connect with the metals in surrounding sediments or water. Often, multitudes of bacteria assemble into a slimy biofilm, which can contain a whole network of nanowires.
The Potential
Adding on electrons causes changes in metals. Instead of being dissolved in water, they form solid compounds. In solid forms, they are less toxic. They don’t spread widely into the environment and can be more easily removed.
Nanowired bacteria could help clean up sites polluted by toxic metals from industry and sewage. In addition, scientists are exploring ways to build bacterial nanowire systems to create fuel cells that use bacteria to generate energy, and bioelectronic devices that can be used to conduct electricity in water.
Destroying nanowire systems could have benefits as well: Medical researchers are investigating ways to knock out disease-causing microbes by disrupting their nanowires.