Catalyze Chemical Breakdown
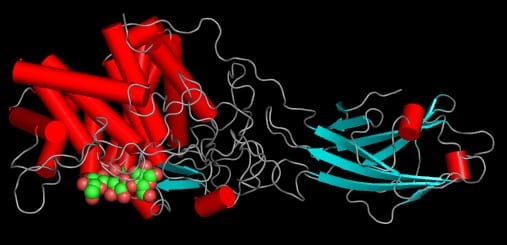
Life depends upon the building up and breaking down of biological molecules. Catalysts, in the form of proteins or RNA, play an important role by dramatically increasing the rate of a chemical transformation––without being consumed in the reaction. The regulatory role that catalysts play in complex biochemical cascades is one reason so many simultaneous chemical transformations can occur inside living cells in water at ambient conditions. For example, consider the 10-enzyme catalytic breakdown and transformation of glucose to pyruvate in the glycolysis metabolic pathway.
Chemically Break Down Polymers
The vast majority of biochemical assembly and breakdown processes–even by the most complex organisms–occur within cells. In fact, cells are able to perform hundreds, even thousands of chemical transformations at the same time under life-friendly conditions (ambient temperature and pressure in an aqueous environment). For example, one of the biochemical pathways that break down proteins into their constituent amino acids is the protease enzyme-catalyzed processes that occur within the confined space of the lysosome–a membrane bound organelle in eukaryotic cells.
Chemically Break Down Organic Compounds
The vast majority of biochemical assembly and breakdown processes–even by the most complex organisms–occur within cells. In fact, cells are able to perform hundreds, even thousands of chemical transformations at the same time under life-friendly conditions (ambient temperature and pressure in an aqueous environment). Part of the reason that decomposition reactions (chemical breakdown) can occur under such mild conditions is because most often, they occur in a stepwise, enzyme-mediated fashion, sipping or releasing small amounts of energy at each step. For example, the breakdown of glucose to pyruvate–and the release of energy–occurs in the 10-step enzyme catalyzed process of glycolysis.